Detection of Foot Sudomotor Dysfunction in Type 1 Diabetes without Clinical Evidence of Peripheral Neuropathy Using SUDOSCAN®

Danyelle Lorrane Carneiro Veloso, Raira Castilho Gomes Nascimento, Eliziane Brandao Leite, Luisiane de Avila Santana, Angelica Amorim Amato.
Article title: Predictors of sudomotor dysfunction in patients with type 1 diabetes without clinical evidence of peripheral neuropathy.
Diabetes Research and Clinical Practice, Volume 170, December 2020, 108500.
AIM: To investigate the frequency of foot sudomotor dysfunction determined by the electrochemical skin conductance test (ESC) and its independent predictors in individuals with type 1 diabetes mellitus (T1D) and no clinical evidence of diabetic peripheral neuropathy (DPN).
METHODS: Adults with T1D for longer than 5 years and without DPN defined by the Michigan Neuropathy Screening Instrument and Neuropathy Disability Score were assessed for foot sudomotor dysfunction by ESC. Multivariate logistic regression analysis was used to examine the association between foot sudomotor dysfunction (ESC < 70 μS) and demographic, clinical, and biochemical variables.
RESULTS: A total of 61 individuals with T1D were included. Their mean age was 29.5 ± 8.6 years, and mean diabetes duration was 17.8 ± 7.9 years. Foot sudomotor dysfunction was present in 16 (26.2%) participants, despite no clinical evidence of DPN. Retinopathy, hand sudomotor dysfunction and glycated haemoglobin (HbA1c) levels were identified as independent predictors of foot sudomotor dysfunction by multivariate logistic regression analysis. Retinopathy, hand sudomotor dysfunction, and every 1% increase of HbA1c increased the odds of foot sudomotor dysfunction by 2.48, 2.82, and 1.24-fold, respectively.
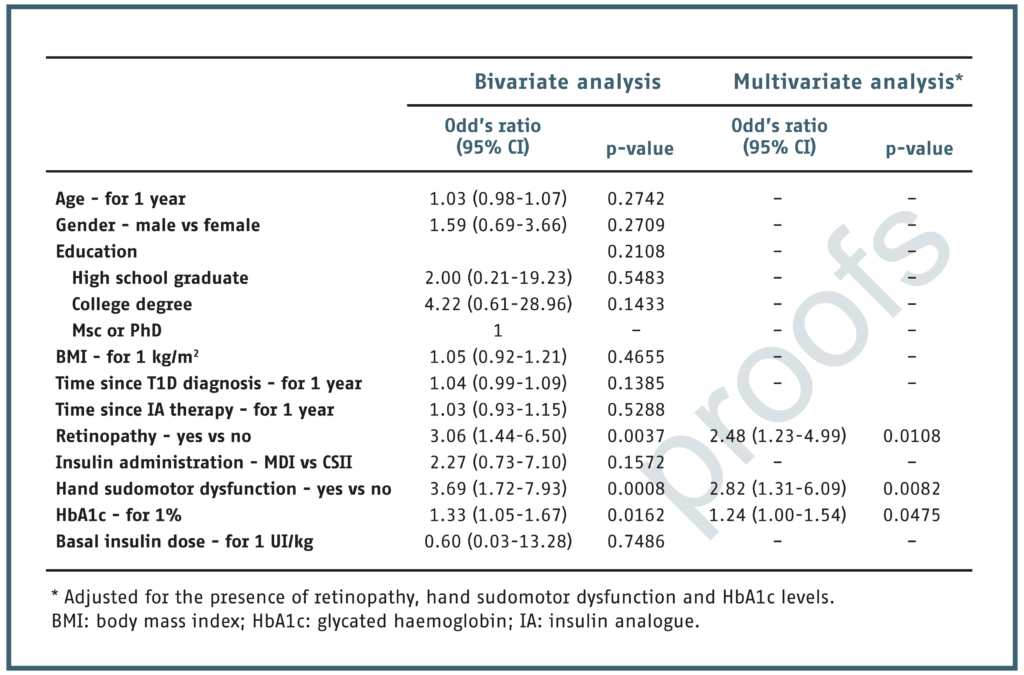
Characteristics of the participants according to the presence of foot sudomotor dysfunction.
CONCLUSION: High frequency of foot sudomotor dysfunction among individuals with T1D and no overt DPN. Retinopathy and higher HbA1c levels, independently predicted sudomotor dysfunction.
ESC assessment is a useful tool in the clinical setting to identify early small-fiber neuropathy.



